The chemical nature of plastic – CONDENSATION POLYMERISATION
In this form of polymerisation, initiation and termination stages do not exist and chain growth occurs by random reaction between two reactive groups. Thus in contradistinction to addition polymerisation an increase in reaction time will produce a significant increase in average molecular weight. An increase in temperature and the use of appropriate catalysts, by increasing the reactivity, will also increase the degree of polymerisation achieved in a given time.
In the case of linear polymers it is often difficult to obtain high molecular weight polymers. The degree of polymerisation & will be given by

If p, the extent of reaction, is the fraction of groups that have reacted, then
When 95% of the groups have reacted (p = 0.95), the degree of polymerization is only 20, indicating a relatively low chain length.
Lower molecular weights are achieved when there is an excess of one reactive group, as it monopolizes chain ends and inhibits further reaction; deliberately adding monofunctional ingredients can similarly control molecular weight. It’s noteworthy that a single condensation reaction can convert two molecules with values of 100 to one molecule with a degree of polymerization (DP) of 200, and reactions between dimers result in tetramers (DP=4), amplifying the impact of individual reactions at later stages despite decreasing concentrations of reactive groups.
Similar to addition polymers, a variety of molecular weights are generated in the formation of molecules during the condensation of bifunctional monomers.
In this context, xw and xn denote the weight average and number average degrees of polymerization. As the reaction nears completion, the ratio between the degrees of polymerization converges towards 2, influencing the approaching molecular weights accordingly.
For trifunctional monomers, the scenario becomes more intricate. Examining the schematic diagrams (Figure 1) reveals that polymers exhibit a greater number of functional groups compared to the monomers.
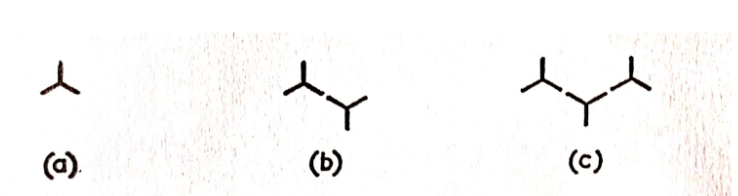
The functionality (number of reactive groups) is observed to be equal to n+2, where n is the degree of polymerization. Consequently, the likelihood of a specific 100-mer (with 102 reactive groups) reacting is more than thirty times greater than that of a specific monomer (with 3 reactive groups). Larger molecules exhibit faster growth, forming increasingly reactive molecules. This phenomenon can lead to the sudden formation of ‘infinitely’ large, cross-linked molecules even when many monomers have not yet reacted. This aligns with the concept of the ‘gel point’ observed in processes involving thermosetting resins. It can be demonstrated that at the gel point in a wholly trifunctional system, xw approaches infinity, while xn is only 4.