The chemical nature of plastic – ADDITION POLYMERISATION (Ionic Polymerisation) – Part 2
Let’s continue with the remaining part.
Please refer the previous part here.
Anionic polymerization, under precise control, can yield a nearly monodisperse polymer sample, where all molecules have the same size. This is in contrast to free radical polymerizations, which, due to the randomness of initiation and termination, result in polydisperse polymers with a wide molecular size distribution. To achieve monodisperse polymers, three conditions must be met:
(1) All the growing chains must be initiated simulyaneously
(2) All the growing chains must have equal growth rates
(3) There must be no transfer or termination reactions so that all chains continue to grow until all of the monomer is consumed.
It follows immediately that the number average degree of polymerisation is given by:
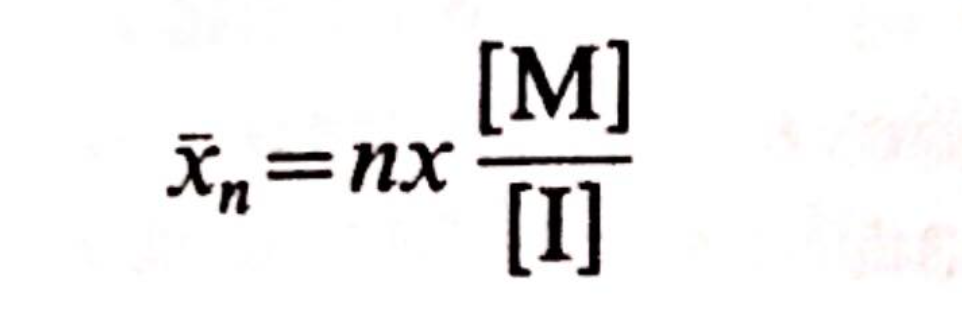
where:
- [M] is the monomer concentration,
- [I] is the initiator concentration,
- n is equal to 1 or 2 depending on whether the initiator forms mono- or di-anions
- x is the fraction of monomer converted into polymer.
In principle, the method can be extended to produce block copolymers with each block being monodisperse, although challenges in avoiding impurities become formidable. Despite this, achieving narrow size distributions, if not monodisperse, is feasible.
An additional feature of anionic polymerization is the ability to couple chains at their ‘living ends.’ When a bifunctional coupling agent is used, it results in a stable non-living linear polymer with an average length approximately twice that of non-coupled molecules. However, with a trivalent coupling agent, T-shaped molecules are formed, and a tetrafunctional agent produces X-shaped molecules. Higher functionality agents lead to the production of star-shaped polymers. An example is the coupling of a butyl-lithium initiated polystyrene with silicon tetrachloride.
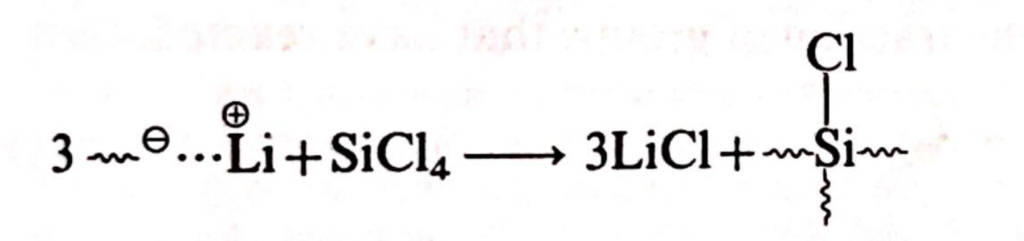
Additional coupling agents include tri- and tetra-chloromethylbenzenes, as well as divinylbenzene.
The system can be utilized for both homopolymers and block copolymers. Examples include some commercial SBS triblock thermoplastic rubbers and closely related K-resins produced by Phillips. Anionic polymerization methods are currently of interest in the preparation of certain diene rubbers.
Due to the contributions of Ziegler in Germany, Natta in Italy, and Pease and Roedel in the United States, the co-ordination polymerization process, related to ionic polymerization, has gained significance. This process is currently employed in the commercial production of polypropylene and polyethylene, and it has also been utilized in the laboratory for synthesizing various novel polymers. The catalyst system employed fundamentally influences the interaction between a monomer and a growing chain, allowing the production of stereoregular polymers.
Stereospecificity in co-ordination polymerization can occur through the formation of a complex between the growing polymer molecule and a catalyst, which is also complexed with a monomer molecule. This specific interaction brings growing polymers and monomers together. The reaction between the growing polymer molecule and the monomer produces a further growing molecule, which then complexes itself with the catalyst, allowing the cycle to be repeated.
The catalysts employed in co-ordination polymerization are complexes formed through the interaction of alkyls of metals in Groups I-III of the Periodic Table with halides and other derivatives of Groups IV-VIII metals. While soluble co-ordination catalysts exist, those utilized for the production of stereoregular polymers are typically solid or adsorbed on solid particles.
Certain metal oxides supported on the surface of inert solid particles can polymerize several olefins. The mechanism of these polymerization reactions is not fully understood but is believed to be ionic in nature. Metal oxides play a role in processes like those of Phillips and Standard Oil for the preparation of polyethylene.