The chemical nature of plastic – Introduction
Defining ‘plastics materials’ precisely can be challenging and may not yield significant value. Still, it’s useful to examine the chemical structure of common polymers, which all share the feature of consisting of large molecules. When considering certain naturally-occurring substances like bitumen, shellac, and amber, their compositions are intricate and varied. However, in all other instances, plastic materials fall under the category of high polymers in the chemical realm. For practical purposes, a polymer can be defined as a large molecule formed by repeating simple units. For instance, polyethylene is primarily a long chain of (CH2) (methylene) groups:
—CH2— CH2— CH2— CH2— CH2— CH2—
The length of these chains can vary, but in commercial polymers, you typically encounter chains containing from 1000 to 10,000 methylene groups. These materials have high molecular weights and are referred to as high polymers or macromolecules. Table “Repeating units of some well-known polymers” hereinafter provides repeating units for various well-known plastics, illustrating the polymer concept.
Apart from plastic materials, many fibers, coatings, and rubbers are essentially high polymers. Nature itself abounds with polymeric materials, such as proteins, cellulose, starch, lignin, and natural rubber, all of which have complex and intricate structures. In comparison, man-made synthetic polymers have relatively simpler molecular architectures.

In addition polymerization, a monomer, a small molecule with a double bond, undergoes a process where the double bond is broken, and the resulting free valences connect with similar molecules. For instance, poly(vinyl chloride) forms when the double bonds in vinyl chloride molecules open up and bond together.

In these instances, the monomer is transformed into a polymer without generating any byproducts. This method is employed for key thermoplastics like polyethylene (a polymer of ethylene), polystyrene (a polymer of styrene) and poly(methyl methacrylate) (a polymer of methyl methacrylate).

In the previous cases, polymerization occurs through the cleavage of carbon-carbon double bonds. It’s also feasible to break carbon-oxygen double bonds and carbon-nitrogen triple bonds. For instance, formaldehyde can polymerize to form polyformaldehyde, also called polyoxymethylene and polyacetal.

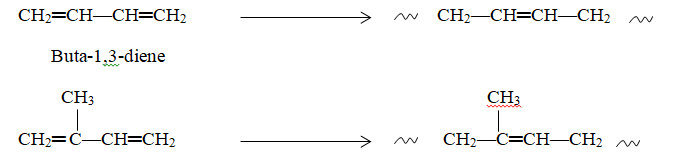
Another variation in double-bond polymerization involves conjugated dienes, which can form long-chain molecules with remaining double bonds within the chain. Buta-1,3-diene and isoprene are well-known examples, leading to 1,4-polybutadiene and 1,4-polyisoprene, as seen in Figure 2.4. Natural rubber corresponds to the formula of 1,4-polyisoprene.
Another method is condensation polymerization, illustrated in the production of linear polyesters. In this process, a dibasic acid reacts with a dihydroxy compound, like a glycol (see Figure 1.5). Here, each acid group combines with a hydroxyl group, resulting in the removal of water to create an ester bond. As each molecule has two reactive ends, long-chain molecules are gradually formed. Unlike addition polymerization, condensation polymerization involves the release of a small molecule during the reaction.
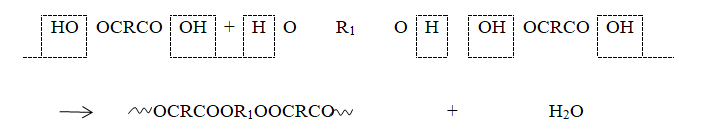
Additionally, it’s not necessary for the monomer to have a double bond. Two more instances of condensation polymerization can be seen in the production of polyamides and polysulfides (see Figure 1.6).
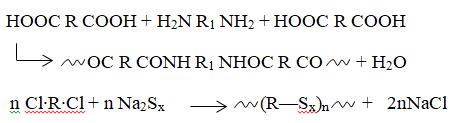
In the first scenario, a dibasic acid reacts with a diamine to create a polyamide, as exemplified by the production of nylon 66 from the combination of adipic acid and hexamethylenediamine.
Although water is often the small molecule released, it’s not always the case. For instance, in the synthesis of polysulfides from dihalides and sodium polysulfide, sodium chloride is generated. The third method for synthetic polymers holds somewhat less commercial significance. This route lacks a universally accepted description but is commonly referred to as rearrangement polymerization and polyaddition.
This process lies between addition and condensation polymerization in several aspects. Similar to the former method, no molecules are eliminated, but its kinetics are akin to the latter. An example includes producing polyurethanes by combining diols (di-alcohols, glycols) with di-isocyanates (see Figure 1.7).
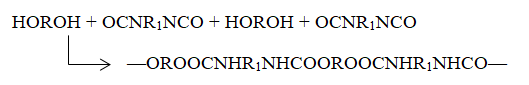
This reaction is crucial in the production of urethane foams.
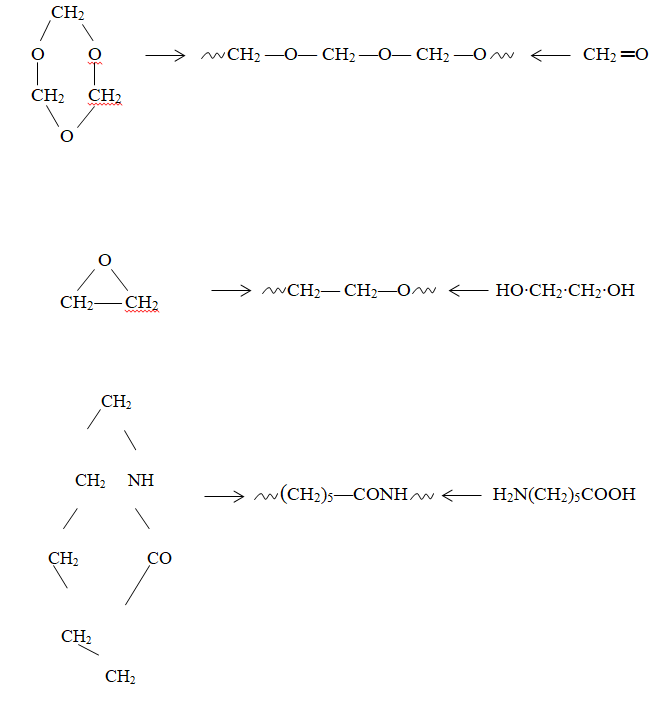
Another variation of rearrangement polymerization is ring-opening polymerization. Key instances include the polymerization of trioxane, ethylene oxide, and ε-caprolactam (refer to Figure 1.8 (a) to (c) respectively). It’s noteworthy that in these cases, the kinetics are more similar to double bond polymerization. An interesting aspect of these three examples is that the same polymers can also be created using other methods, with the first through addition polymerization and the second and third through condensation processes.
It’s worth mentioning that several commercial polymers are manufactured by chemically modifying other polymers, whether they are natural or synthetic. For instance, cellulose acetate is derived from the naturally-occurring polymer cellulose, and polyethylene (Hypalon) originates from polyethylene.